Infant BIKA®
Description :
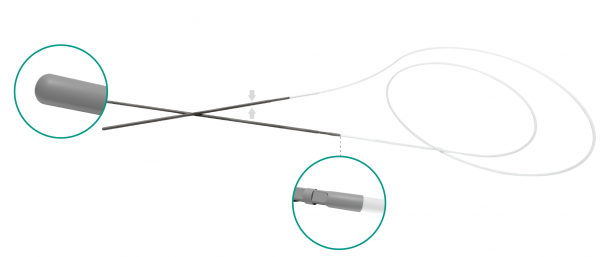
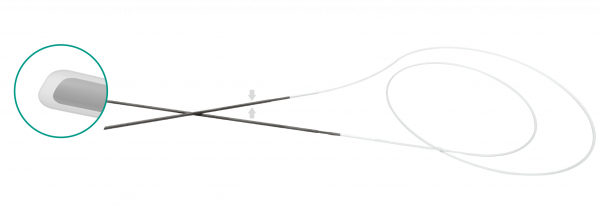
FCI BIKA® intubation systems have been the first models designed for bicanalicular intubation, especially in case of congenital nasolacrimal duct obstruction and dacryocystorhinostomy (DCR). The metallic probes are securely connected at the silicone tube for an easy and safe retrieval fromthe nose.
Main characteristics :
• Designed by J.-A. Bernard, M.D.
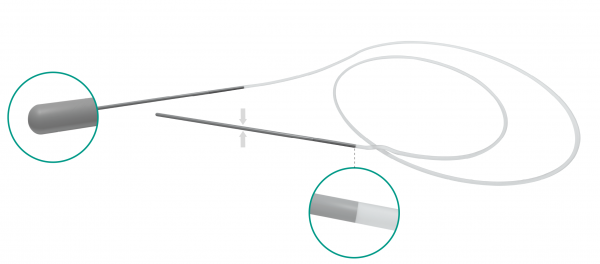
• Strong connection between silicone tube and metallic probe
• Non-traumatic rounded tip • 0.64 mm silicone tube / 55 mm long & 0.4 mm diameter metallic probe
• Probe embedded in silicone tube / Perfectly smooth junction
• Indicated for newborn
• Medical grade silicone
• Sterile