EZYPOR®
Description :
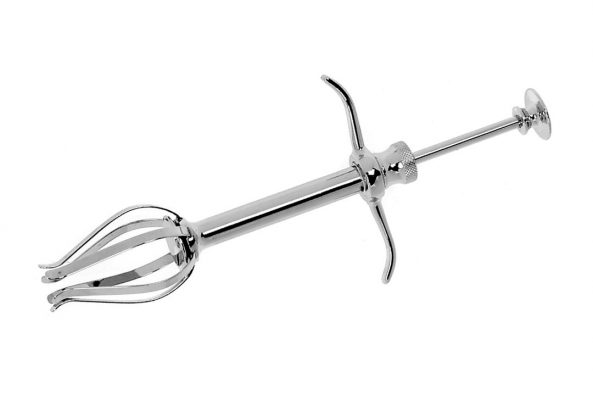
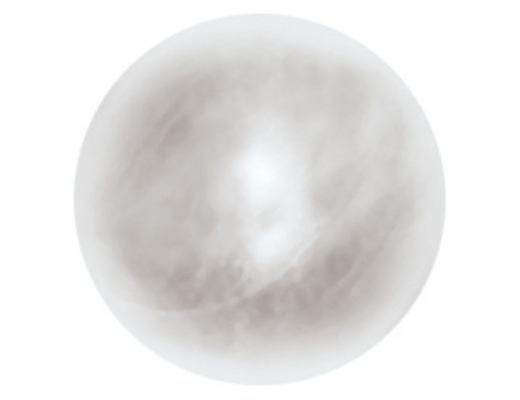
EZYPOR® is FCI ultimate high density polyethylene orbital implant with innovative suturing platform.
EZYPOR® has a smooth anterior suturing surface with 8 suture tunnels allowing multiple suturing options.
The use of FCI sizer set (S6.3060) is recommended to select the appropriate implant diameter which is essential to reach the utmost final cosmetic result. Then, EZYPOR® is gently inserted into the eye socket with a sphere introducer (S6.3050).
Main characteristics :
• Indicated for enucleation, evisceration and secondary implantation
• Made of Ultra High Molecular Weight PolyEthylene (UHMWPE)
• Porosity between 40 and 60% for optimum colonization of fibrovascular tissues
• Smooth anterior suturing platform with 8 suture tunnels to facilitate needle insertion and muscles fixation
• No need for autologous graft
• Sterile
*EZYPOR® 12 mm and 14 mm do not have suturing platform.